IgM/IgG Antibody (CLIA) Detection Application Introduction
Novel Coronavirus (2019-nCoV) Antibody Detection Background
- At the end of December 2019, the World Health Organization officially named the novel coronavirus that caused the pneumonia epidemic in Wuhan City as “2019 Novel Coronavirus (COVID-19)” .
- Since the outbreak of novel coronavirus infection pneumonia, the situation has been more and more severe. As of 14:00 on March 24, a total of 81,747 confirmed cases and 3,283 deaths have been reported nationwide; a total of 292,396 and 13,017 deaths have been confirmed abroad.
Novel Coronavirus (2019-nCoV) Detection Method
- In the early stages of the outbreak, laboratory detection methods mainly relied on nucleic acid detection and imaging. However, nucleic acid testing has its own limitation and the limitaiton on sampling. It is difficult to carry out large-scale tests and has a high rate of missed detection. CT signs are non-specific;
- The antibody detection can effectively compensate for the risk of missed detection of nucleic acid detection, facilitate the rapid determination of infection people, and help the overall judgment and control of epidemic on nationalwide and worldwide.
| Detection | Methodology | Detecting Principle | Advantages | Disadvantages |
|---|---|---|---|---|
| Etiology Detection | Nucleic acid detection Viral gene sequencing |
•Specimens tested positive for new coronavirus nucleic acid; •Specimen virus gene sequencing, highly homologous to known new coronaviruses; |
Confirmed indicators | •Need a professional PCR laboratory; •High risk of sample collection, false; negatives due to low sample virus copy numbers; •Slow speed, sample processing and testing time> 3 hours; •Low flux; •Complex operation and high professional; requirements for personnel; •Easy to cause operator infection. |
| Imaging Detection | CT | Find typical ground glass-like features | Determining whether there is inflammation in the lungs, which has an auxiliary diagnostic value for pneumonia | •CT signs are non-specific and similar to other viral pneumonia; •No obvious lung imaging changes in early infection. |
Clinical Significance and Necessity of Antibody Detection for Novel Coronavirus (2019-nCoV)
Novel Coronavirus (2019-nCoV) Serological Analysis
Serological evidence: detection of specific antibody IgM & IgG
- Specificity IgM and IgG antibody are the human immune response to the virus (cause). The antibody (result) produced are indirect evidence that the human is currently infected or previously infected with the virus.
- Coronavirus IgM antibody specificity begins to appear positive after 3-5 days of onset. IgG appears later than IgM, but the duration is significantly longer than IgM. The recovery period of IgG antibody titers is 4 times or more higher than that of the acute phase.
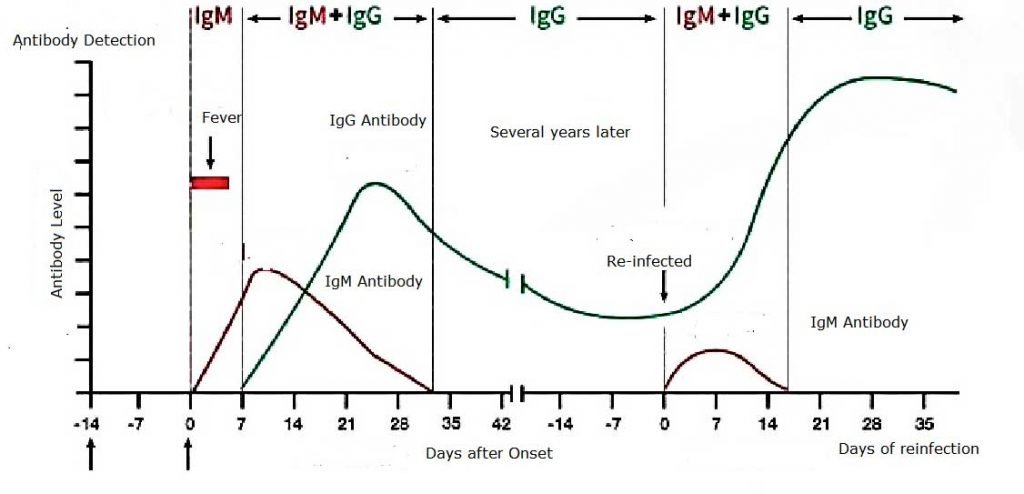
Clinical Data Evaluation
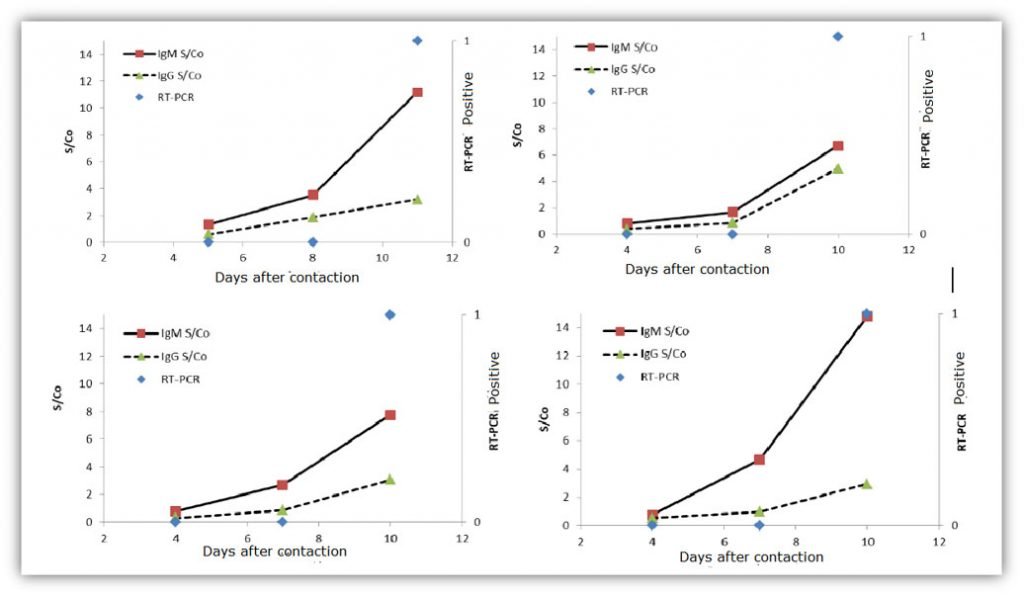
Clinical significance of Antibody Detection
- The testing data shows that the IgM antibody detection, sampling is simple and safe, and the detection rate of the Novel Coronavirus (2019-nCoV) is higher than that of the nucleic acid, which helps to shorten the diagnosis time of close contact or suspected patients.
- Screening of high-risk population (compared to PCR technology, it can truly achieve fast and high-throughput accurate screening).
- Improving the accuracy of virus detection (the current PCR technology has false negative).
- Surveillance and effect evaluation of patients undergoing plasma treatment during recovery.
- IgG can be used for epidemiological investigation.
- Used in blood stations and blood banks to ensure blood safety.
- Vaccine titer assessment.
- Novel Coronavirus Pneumonia Diagnosis and Treatment Plan (Trial Version 7) “Serological Diagnosis Basis”.
Bioscience Novel Coronavirus (2019-nCoV) Diagnostic Kit
Registration No .: National Machinery Standard 20203400182 / National Machinery Standard 20203400183
Magnetic particle chemiluminescence
Reaction principle: indirect chemiluminescence
High sensitivity, high stability, good repeatability, easy operation with fully automated;
Simple sampling: venous blood with is simple, fast and safe to collect;
Precision: CV≤8% within batch, CV≤10% between batches;
Calibrator: The kit comes with a calibrator or a negative/positive reference substance, which does not need to be purchased separately;
One of the basis for the diagnosis of novel coronavirus pneumonia;
Packaging specifications: 100 tests / box, 500 tests / box;
Storage conditions and shelf life: more than 6 months at 2-8 ° C (including 6 months).
Bioscience Novel Coronavirus Antibody Detection System
Axceed 260 :
- Through Put: 240 T/H;
- High-polished steel needle loading, carrying pollution <10-6, no additional Tip consumables;
- Expandable assembly line with maximum speed up to 4800 T/H;
- Sample predilution on board;
- High-throughput, large-scale screening;
- Applicable to secondary and above hospital and physical examination institutions;

Bioscience CLIS alpha:
- Fast:Immunoassy/Chemistry Expandable assembly line up to 20 units,throughput reaches up to 4800T/H & 16000T/H;
- Flexxible Scalabiliy:Meet the various laboratory layouts with good site adaptability;
- RFID Application:Track samples in real time to avoid orbital congestion;
- High efficiency: Rapid diagnosis and re-inspection, which shorten the reporting time of detection;
- Large sample storage capacity: the upload / download module can be expanded, and 288 * N / 360 * N samples can be loaded at one time, supporting online adding;
- Applicable to second-level and above hospitals, Individual Labs and Physical Examination Centers;

Interpretation of the Novel Coronavirus (2019-nCoV) Antibody Detection Policy
- Serum novel coronavirus specific IgM antibody and IgG antibody were positive;
- Serum novel coronavirus-specific IgG antibody changed from negative to positive or coronavirus-specific IgG antibody during the recovery period is 4 times higher than that during the acute period.
"Four Early Detection" Solution for Novel Coronavirus (2019-nCoV) Pneumonia
Early detection and reporting:
- Specimen collection and laboratory testing: Medical institutions collect clinical specimens of cases (see the Specimen Collection and Testing Section of the Novel Coronavirus Pneumonia Prevention and Control Plan (Fifth Edition) for details) and send them to the local designated disease control institution or medical institution or Testing performed by a third-party testing laboratory. After receiving the specimens, the institution undertaking the testing should start the testing immediately, complete and feedback the test results within 24 hours
- Environmental specimen monitoring and sero-epidemiological investigations: carried out in a timely manner according to actual needs.
Full implementation of “four early, four concentrated”.
- China implements the “four early epidemic prevention principles” of early detection, early isolation, early diagnosis, and early treatment.
- Allow clinicians to detect with a lower suspicion index.
Novel Coronavirus (COVID-19) Serological Analysis
- Rapid detection of IgM and IgG antibody is also important to facilitate early diagnosis;
- Priority of thorough case screening and detection;
- Quick search and isolate the patient;
Methodological Comparison of Novel Coronavirus (2019-nCoV) Antibody Detection
Antibody Detection Methodological Comparison
| Detection Category | Detection Principle | Advantage | Disadvantage |
|---|---|---|---|
| IgM/IgG Detection Separately | Bioscience Magnatic Particle Chemiluminescence immunoassay method |
1. IgM & IgG detection separately, provide the quantitative result(S/Co), to assist to identify the dynamic fluctuations of the Antibodies, which will help clinicians accurately identify the progress of a patient’s infection; (IgM infection appears early and rises rapidly, while it rapidly decreases to a low level at the recovery period. IgG appears later than IgM and then rises rapidly after recovery. IgG at the recovery period is generally more than 4 times higher than the acute period, and it exists in the body for a long time. 2. IgG facilitates epidemiological investigations (exploring patterns of transmission and incidence of novel coronaviruses to develop control strategies); 3. It used for mass screening of suspected cases and extensive contacts; 4. It is one of the foundations of the seventh edition of the Novel Coronavirus diagnosis and treatment plan. 5. Chemiluminescence Immunoassay method has a very high sensitivity, which helps identify early changes in antibody and helps “early detection.” |
Interference from non-specific antibody in individual blood samples (such interference has been significantly reduced by diluted sample or using the reagents with cleaning antibody). |
| Colloidal Gold |
easy to operation | 1. Low sensitivity. 2. May existing Big error for weak positive samples on results reading. 3. False positives caused by interfering substances. 4. Qualitative, not quantitative, it is hard to track antibody dynamically changes, either to identify the progress of patient infection. |
|
| Antibody(IgG+IgM) | Colloidal Gold |
easy to operate |
1. Low sensitivity, requiring multiple detections of suspicious samples. 2. May existing Big error for weak positive samples on results reading. 3. False positives caused by interfering substances. 4. Qualitative, not quantitative, it is hard to track antibody dynamically changes, either to identify the progress of the patient infection. 5. It cannot distinguish IgM and IgG, when detected a positive result, it is still necessary to do a further detection of IgM & IgG separately. 6. Not the Confirmed diagnosis standard of Novel Coronavirus (2019-nCoV). |
| Chemiluminescence immunoassay method | one test only | 1. It cannot distinguish IgM and IgG when detected a positive result, it is still necessary to do a further detection of IgM & IgG separately. 2. Inability to help clinicians identify patient infection processes; 3. Not the Confirmed diagnosis standard of Novel Coronavirus (2019-nCoV). |
Novel Coronavirus (2019-nCoV) Antibody Detection Method Comparison
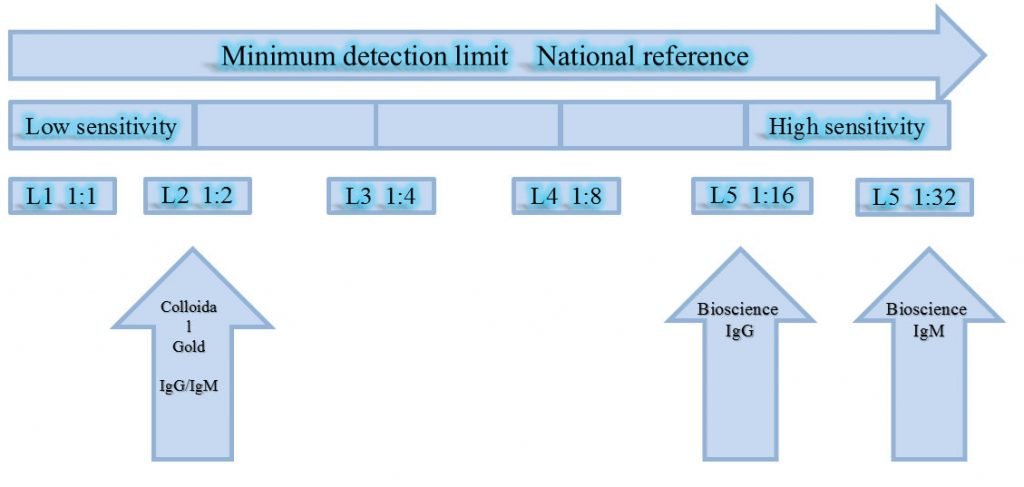
Comparison of Antibody Detection Method

- The sample is a nucleic acid positive sample;
- The results of Antibody from the colloidal gold method and chemiluminescence method is showing that: the sample which detected as negative with Colloidal Gold method is actually weak positive, which shows a low sensitivity
Bioscience Clinical Data Evaluation
- IgM and IgG are tested separately (IgM is an indicator of early infection, it reduced to negative at the recovery period, and IgG is an indicator of mid-to-late infection, which will last for a long time). The antibody dynamic fluctuation data helps clinicians accurately identify the progress of patient infection.
- IgG positive help the epidemiological investigation.
- High detection sensitivity is good for early diagnosis.
- Till now, the antibody kit has been tested in a total of 13,532 samples in Hubei Province, Chongqing City and other places, of which 1,362 were confirmed cases of Novel Coronavirus (2019-nCoV). The results show that:
The combined detection of IgG & IgM gives the Sensitivity of 94.33%, and the specificity of 99.5%.
Clinical Requirement for Antibody Detection
- Based on the comparison of the advantages and disadvantages of the above Novel Coronavirus(COVID-19) detection methods, it can be seen that the serological detection method is operation easily, which is fast and safety with high sensitivity, high stability, good repeatability, and high throughput.
- Novel coronavirus (2019-nCoV) lgM / lgG antibody detection can identify the antibody dynamic fluctuations, help clinicians to accurately identify the progress of the patient infection, and serve as one of the bases for the diagnosis of novel coronavirus pneumonia cases.
- It is helpful for the early diagnosis of novel coronaviruses, and it is of great significance for controlling the spread of epidemics and treating patients.
Current Problems
- Commodity price and medical insurance: Except Chongqing City, at present, there are no novel coronavirus (2019-nCoV) lgM / lgG antibody detection prices and medical insurance in all provinces and cities across the country, which has hindered the application and promotion of this product. Nucleic acids are mainly purchased through the CDC. Allocate to medical institutions at all levels. If 2019-nCoV lgM / lgG antibody detection can be uniformly purchased through the CDC, the application will increase on a large scale.
- Unlicensed products disrupt the market: Vicious competition for products without a registration certificate from the State Food and Drug Administration is widespread in medical institutions at all levels.
- Cognitive issues: Since the 7th edition of the National Guidelines for Diagnosis and Treatment was issued on March 4, it has been confirmed that lgM / lgG antibody are a simultaneously positive or 4-times increase in lgG antibody can be used as indicators for diagnosis, but there are cognitive awareness at medical institutions or experts at all levels Preconceived, and no change in concept has hindered application.
- To a certain extent, there are cases where it is as unwilling to detect new cases of infection as novel coronavirus.
Suggestions
- Approve commodity prices and medical insurance in each province as soon as possible.
- Inventory of undocumented products.
- lThe promotion of double detection of nucleic acids and lgM / lgG antibody is conducive to improving the detection rate, especially in cases where customs-imported novel coronavirus disease is still severe.
- Included In a routine screening of fever clinics at medical institutions at all levels to avoid serious consequences caused by missed or misdiagnosed cases.
- Help to prevent and control overseas epidemics, expanding the overseas market.
